Precision Medical Device CNC Machining Services
XTJ delivers ISO 13485-certified CNC machining with over 20 years of expertise in medical component manufacturing. We combine high precision tolerances, full regulatory compliance, and rapid turnaround times—with no minimum order requirements. Save up to 30% on manufacturing costs while ensuring exceptional quality and patient safety through our advanced machining capabilities.
-
Precision Tolerances
Exceptional accuracy for critical medical components -
On-Time Delivery
Reliable production schedules you can trust -
Minimum Order
Full flexibility from prototype to production -
Days for Prototypes
Rapid turnaround for development cycles

Uncompromising Precision and Quality Standards
The life-critical nature of medical devices demands exceptional precision and uncompromised quality. Any deviation in manufacturing tolerances could lead to device failure and jeopardize patient safety. At XTJ, we recognize this responsibility and implement rigorous quality protocols at every production stage.
We double-check engineering drawings and machining specifications throughout the entire process—from initial prototype development through low-volume validation runs to high-volume production. During prototyping, our engineering team collaborates closely on design iterations to ensure flawless execution before committing to production tooling.
Our management considers overlooking quality details completely unacceptable. This commitment guarantees you receive standardized, reliable parts that meet your exact specifications every time, maintaining consistency across production batches.

Deep Technical Expertise
20+ years specializing in medical device precision machining with proven capabilities in complex geometries
Tailored Solutions
Custom manufacturing approaches aligned perfectly with your unique device requirements
Your Success Defines Ours
We measure our performance by your product success and market acceptance
Material Compliance and Regulatory Certainty
Ensuring materials meet rigorous regulatory standards is non-negotiable for medical device manufacturing. Biocompatibility, sterilization compatibility, and full material traceability are essential requirements. Our material selections strictly comply with FDA regulations, ISO 13485 standards, and CE Marking requirements to safeguard device performance and patient well-being.
XTJ sources materials exclusively from trusted domestic suppliers who meet stringent global safety benchmarks. For specialized applications requiring premium grades, we import raw materials directly from certified European and US suppliers, complete with comprehensive certification documentation including MSDS and RoHS/REACH compliance reports.
Titanium Alloys
Ti-6Al-4V Grade 5 & 23 ELI: Biocompatible, lightweight, and high-strength—ideal for implants, prosthetics, and
load-bearing medical components requiring excellent corrosion resistance.
Stainless Steel
316L, 17-4PH: Superior corrosion resistance and sterilization compatibility make these grades perfect for surgical
instruments and reusable medical tools.
Cobalt Chrome
CoCrMo: Exceptional wear resistance and biocompatibility for demanding applications like joint replacements
and dental framework structures.
Engineering Polymers
PEEK & UHMWPE: Radiolucent materials with bone-like modulus for spinal cages, orthopedic applications,
and applications requiring MRI compatibility.
Aluminum Alloys
6061, 7075: Cost-effective solutions for non-implant device housings, enclosures, and structural components
requiring lightweight strength.
Medical Plastics
PC, ABS, POM: Quick-prototype options ideal for device enclosures, covers, and non-critical components with excellent dimensional stability.
- All materials include complete traceability documentation and quality certificates, eliminating material-related compliance risks and giving you full confidence when launching products to market.
Material Compliance and Regulatory Certainty
Medical device components demand meticulous precision, and their intricate designs frequently exceed the capabilities of standard 3-axis CNC machines. XTJ’s state-of-the-art manufacturing facility features comprehensive machining capabilities including 3-axis, 4-axis, and 5-axis CNC equipment to handle diverse specifications and complex geometries with exceptional accuracy.
5-Axis CNC Machining Advantages
Five-axis CNC machining revolutionizes complex medical device production by enabling unmatched precision, dramatically minimized setup times, and highly efficient multi-angle processing capabilities. This technology proves ideal for orthopedic implants, surgical instruments, and components requiring intricate contours or internal features.
- Complex Geometry Access Machine intricate angles and undercuts in single setups
- Reduced Setup Time Fewer repositioning operations mean faster throughput
- Superior Surface Finish Continuous tool engagement creates smoother surfaces
- Enhanced Precision Minimized errors from multiple fixture changes
Mastering Complex Geometries
From spinal implants and hip prosthetics to specialized surgical instruments, intricate medical parts with challenging geometries require advanced CNC techniques, sophisticated CAM programming, and expert tooling knowledge. XTJ’s engineering team ensures seamless execution for even the most demanding component designs.
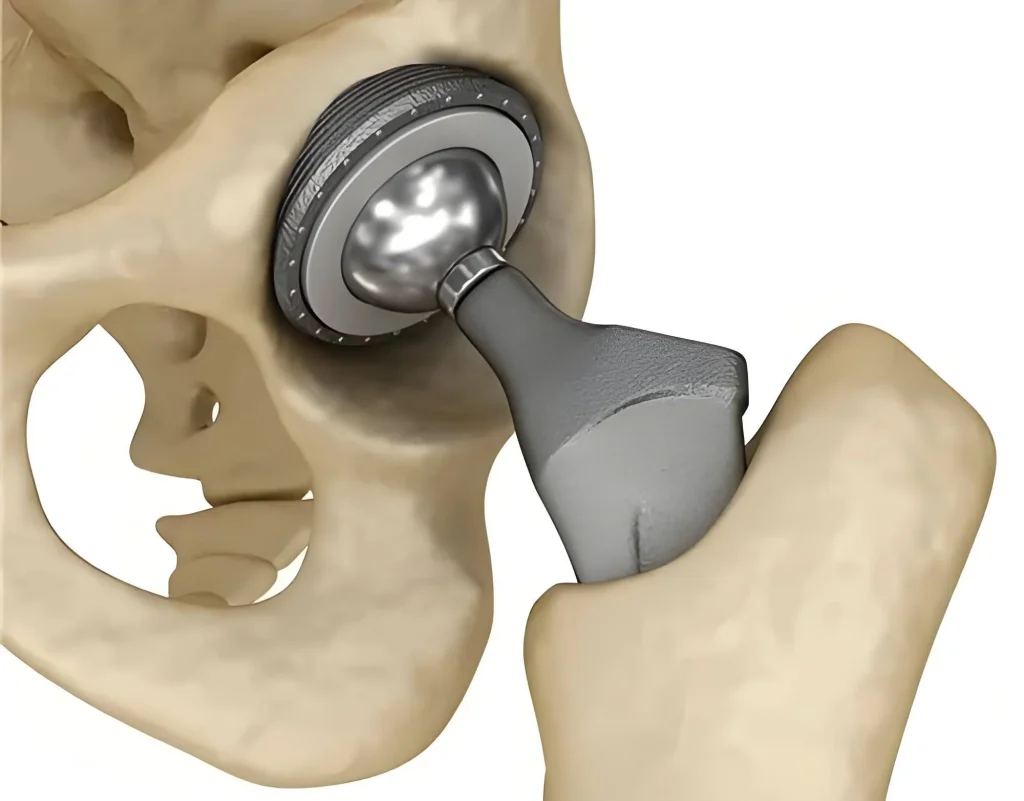
Our adaptive machining strategies optimize tool paths and cutting parameters to reduce production costs while maintaining exceptional dimensional accuracy and surface quality standards.
Accelerated Lead Times Without Quality Compromise
Medical innovators and OEMs frequently require rapid turnaround capabilities to meet aggressive market launch timelines or accelerate R&D cycles—without ever sacrificing quality standards or regulatory compliance. Speed to market can determine competitive advantage in the medical device industry.
XTJ’s integrated order management system keeps your project on track from quotation through final delivery. Our sales teams monitor production progress in real-time through digital dashboards, providing complete transparency and proactive updates throughout manufacturing. We leverage automation and advanced scheduling to maximize efficiency while maintaining our rigorous quality protocols.
This systematic approach ensures deliveries stay firmly within your timeline expectations, supporting everything from urgent prototype iterations to scaled production runs with just-in-time delivery options for inventory optimization.
Quotation Response
4–24 hours for detailed pricing
Prototype Production
3–7 days for functional samples
Low-Volume Runs
10–18 days for validation batches
High-Volume Production
3–6 weeks with JIT available
Accelerated Lead Times Without Quality Compromise
Medical innovators and OEMs frequently require rapid turnaround capabilities to meet aggressive market launch timelines or accelerate R&D cycles—without ever sacrificing quality standards or regulatory compliance. Speed to market can determine competitive advantage in the medical device industry.
Design for Manufacturability
Our engineering team provides comprehensive DFM reviews to streamline your device designs, identifying opportunities to reduce material waste, minimize machining complexity, and optimize production efficiency while maintaining all functional and regulatory requirements.
Advanced Automation
Automated workflows and robotic material handling minimize manual intervention throughout production, dramatically boosting consistency and repeatability while slashing labor costs across high-volume manufacturing runs without compromising quality standards.

Intelligent Material Selection
We recommend compliant materials that strike the optimal balance between clinical performance and budget constraints, avoiding costly over-specification while ensuring all biocompatibility and mechanical property requirements are fully met for your application.
Rigorous Quality Control
In-process inspections and statistical process control minimize defects, eliminate costly rework, and reduce scrap rates—maximizing production yield and driving down per-unit costs while ensuring every part meets exacting specifications and regulatory standards.

Uncompromising Precision and Quality Standards
In medical device innovation, sharing sensitive designs and proprietary technology with manufacturing partners demands ironclad confidentiality and trust. Your intellectual property represents years of research investment and competitive advantage—protecting it is absolutely paramount.
XTJ prioritizes your IP security as a foundational business principle. We sign comprehensive Non-Disclosure Agreements as standard practice with every client engagement, legally committing to never disclose, replicate, or misuse any proprietary information without your explicit written permission.
Our facility maintains strict access controls, secure file management systems, and employee confidentiality training protocols. This trust-based approach creates the foundation for seamless collaboration, letting you focus confidently on breakthrough innovations while we handle manufacturing execution with complete discretion.
Deep Technical Expertise
20+ years specializing in medical device precision machining with proven capabilities in complex geometries
Tailored Solutions
Custom manufacturing approaches aligned perfectly with your unique device requirements
Your Success Defines Ours
We measure our performance by your product success and market acceptance
Trusted by Leading Medical Innovators
We’re proud to support pioneering medical device companies and healthcare innovators worldwide. Our partnerships with industry leaders demonstrate our capability to meet the most demanding technical specifications and regulatory requirements while delivering exceptional quality and reliability.
Through our advanced technology center, we collaborate with global medical technology leaders including LEPU Medical, Yuwell Medical, Haier Biomedical, and United Imaging Medical. These strategic partnerships drive peak manufacturing performance, consistently exceed expectations, and deliver precise solutions through unmatched craftsmanship and engineering expertise.
Elevate Your Medical Devices with XTJ Precision
Partner with XTJ for compliant, high-quality CNC machining solutions that accelerate your development timeline and reduce manufacturing costs. Our ISO 13485-certified facility combines decades of medical device expertise with cutting-edge technology to deliver the precision, quality, and reliability your innovations demand.
Full Regulatory Compliance
ISO 13485, FDA, and CE certified manufacturing with complete documentation
Rapid Turnaround
Prototypes in 3-7 days with scalable production capabilities
Cost Optimization
Save 20-40% through DFM expertise and efficient processes
IP Protection
Comprehensive NDAs and secure systems safeguard your innovations
No Minimum Orders
Complete flexibility from single prototypes to high-volume production
Expert Support
Dedicated engineering assistance throughout your project lifecycle
From concept validation through full-scale manufacturing, XTJ provides the precision machining partnership you need to bring life-changing medical devices to market faster and more cost-effectively. Experience the difference that true medical manufacturing expertise makes.
Frequently Asked Questions
Here are answers to some of the most common questions about medical device machining, based on our expertise at XTJ Precision Manufacturing.
Common choices include titanium alloys (Ti-6Al-4V), medical-grade stainless steel (316L, 17-4PH), cobalt chrome, aluminum alloys, and high-performance engineering polymers like PEEK. Materials are selected based on biocompatibility, sterilization compatibility, corrosion resistance, and mechanical properties required to meet essential FDA and ISO medical standards.
Five-axis machining enables complex three-dimensional shapes and intricate features with superior precision and consistency. It dramatically reduces setup time by accessing multiple angles in a single operation, improves surface finish quality, and enhances overall production efficiency—making it perfect for complex implants, surgical instruments, and components with challenging geometries.
All XTJ-manufactured medical components meet ISO 13485 quality management standards, FDA regulations for medical device manufacturing, and CE Marking requirements for European markets. We provide complete documentation packages including material certificates, inspection reports, and traceability records to support your regulatory submissions and quality assurance processes.
CNC machining delivers exceptional precision, excellent repeatability, and minimal material waste compared to traditional manufacturing methods. The process accelerates development timelines, reduces tooling costs for low to medium volumes, and eliminates expensive secondary operations. At XTJ, optimized processes and DFM expertise often save clients up to 30% compared to alternative suppliers.
Swiss-type CNC machines excel at producing ultra-precise micro components as small as 0.5mm diameter with exceptional concentricity and surface finish. The guide bushing support and sliding headstock design deliver tight tolerances and superior accuracy—making this technology ideal for catheter components, micro-implants, dental instruments, and other miniature medical parts requiring extreme precision.
